Modern life depends on electricity and electrical devices, from cars and buses, to phones and laptops, to the electrical systems in homes. Behind many of these devices is a type of energy storage device, the supercapacitor. My team of engineers is working to make these supercapacitors even better at storing energy by studying how they store energy at the nanoscale.
Supercapacitors, like batteries, are energy storage devices. They charge faster than batteries, often within a few seconds to a minute, but generally store less energy. They are used in devices that require storing or delivering energy in a short amount of time. In the car and in elevators, they can help recover energy during braking to slow down. They help meet fluctuating power demand in laptops and cameras and stabilize power loads on power grids.

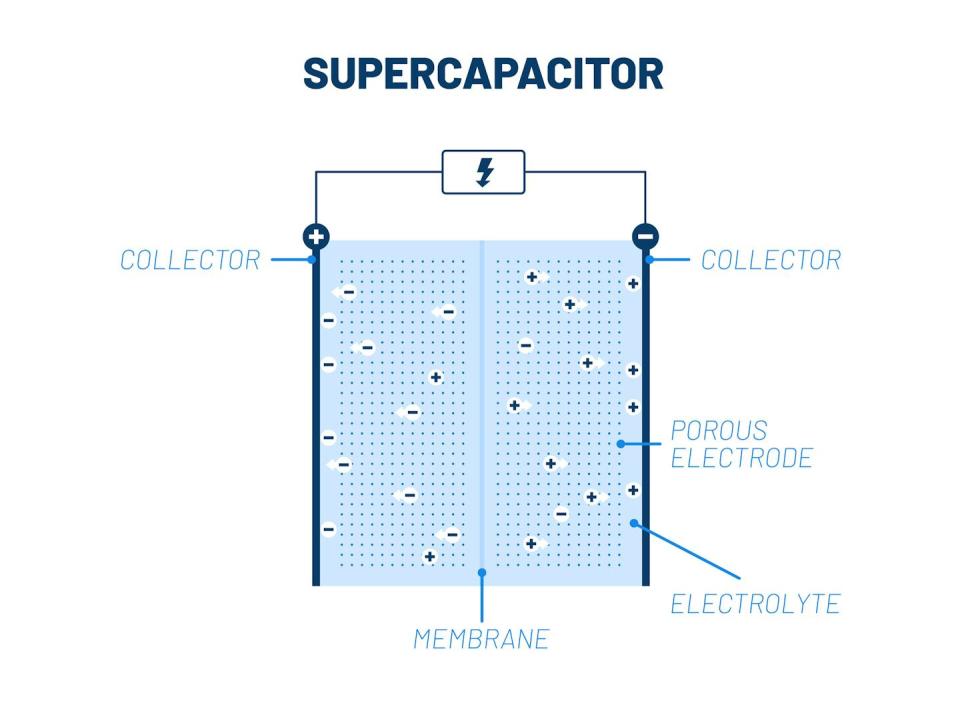
Batteries operate through reactions in which chemical species donate or receive electrons. Supercapacitors, on the other hand, do not rely on reactions and are like a charge sponge. When you dip a sponge in water, it absorbs the water because the sponge is porous – it contains empty pores where water can be absorbed. The best supercapacitors They absorb the largest load per unit volume, which means they have a high energy storage capacity without taking up much space.
In published research in the Proceedings of the National Academy of Sciences in May 2024, my student Filipe Henriquecollaborator Pawel Zuk and me describe how ions move in a network of nanopores, or tiny pores just nanometers wide. This research could one day improve the energy storage capabilities of supercapacitors.
All about pores
Scientists can increase capacitance of a material, or the ability to store charge, making its surface porous at the nanoscale. A nanoporous material can have a surface area of up to 20,000 square meters (215,278 square feet) – the equivalent of about four football fields – in just 10 grams (one-third of an ounce) of weight.
Over the past 20 years, researchers have studied how control this porous structure and the flow of ions, which are tiny charged particles, through the material. Understanding ion flow can help researchers control the rate at which a supercapacitor charges and releases energy.
But researchers still don’t know exactly how ions flow in and out of porous materials.
Each pore in a sheet of porous materials it is a small hole filled with positive and negative ions. The pore opening connects to a reservoir of positive and negative ions. These ions come from an electrolyte, a conducting fluid.

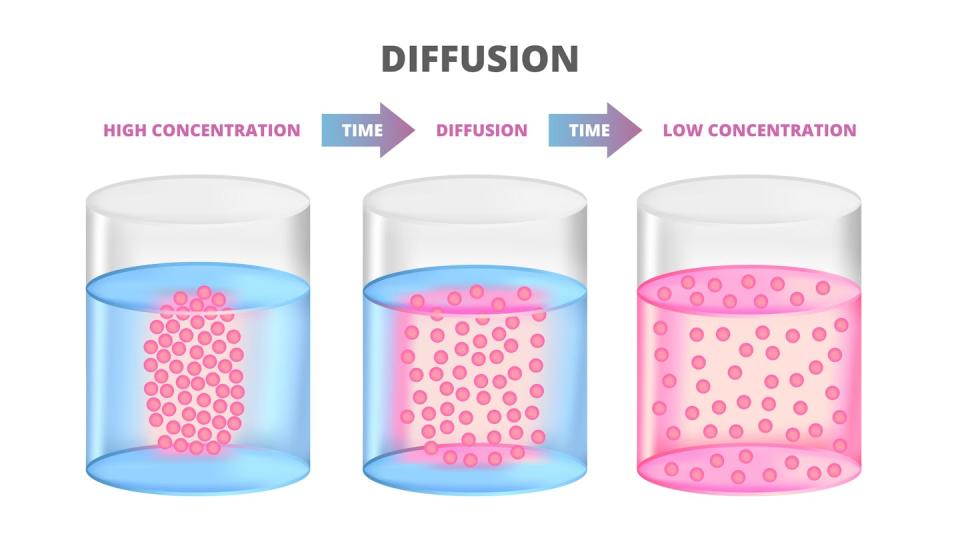
For example, if you put salt in water, each salt molecule separates into a positively charged sodium ion and a negatively charged chloride ion.
When the pore surface is charged, ions flow from the reservoir to the pore or vice versa. If the surface is positively charged, negative ions flow from the reservoir into the pore and positively charged ions leave the pore as they are repelled. This flow forms capacitors, which hold the charge in place and store energy. When surface charge is discharged, ions flow in the reverse direction and energy is released.
Now, imagine a pore divided into two different branched pores. How do ions flow from the main pore to these branches?
Think of ions as cars and pores as roads. Traffic flow on a single road is simple. But at an intersection, rules are needed to avoid accidents or traffic jams, which is why we have traffic lights. and roundabouts. However, scientists don’t fully understand the rules that ions flowing through a junction follow. Figuring out these rules can help researchers understand how a supercapacitor will charge.
Modifying a law of physics
Engineers often use a set of laws of physics called “Kirchoff’s laws”To determine the distribution of electrical current through a junction. However, Kirchhoff circuit laws were derived for electron transport, not ion transport.
Electrons only move when there is an electric field, but ions can move without an electric field, through diffusion. In the same way that a pinch of salt slowly dissolves in a glass of water, the ions move from more concentrated areas to less concentrated areas.

Kirchhoff’s laws are like accounting principles for circuit junctions. The first law says that the current entering a junction must equal the current leaving it. The second law states that voltage, the pressure that pushes electrons through current, cannot change abruptly at an intersection. Otherwise, it would create an extra current and upset the balance.
Because ions also move by diffusion and not just using an electric field, my team modified Kirchhoff’s laws to fit ionic currents. We replace the voltage, V, with an electrochemical voltage, φ, which combines tension and diffusion. This modification made it possible to analyze pore networks, which was previously impossible.
We use modified Kirchoff’s law to simulate and predict how ions flow through a large network of nanopores.
The road ahead
Our study found that Splitting the current from a pore into junctions can slow the rate at which charged ions flow into the material. But that depends on where the division is. And the way these pores are arranged in the materials also affects the loading speed.
This research opens new doors for understanding supercapacitor materials and developing better materials.
For example, our model can help scientists simulate different pore networks to see which best matches their experimental data and optimize the materials they use in supercapacitors.
While our work has focused on simple networks, researchers could apply this approach to much larger, more complex networks to better understand how a material’s porous structure affects its performance.
In the future, supercapacitors could be made from biodegradable materialspower flexible wearable devicesand maybe customizable through 3D printing. Understanding ion flow is a key step toward improving supercapacitors for faster electronics.
This article was republished from The conversation, an independent, nonprofit news organization that brings you trusted facts and analysis to help you understand our complex world. It was written by: Ankur Gupta, University of Colorado at Boulder
See more information:
Ankur Gupta receives funding from NSF.
















/cdn.vox-cdn.com/uploads/chorus_asset/file/25199378/HT012_Google_drive.png?w=300&resize=300,300&ssl=1)


/cdn.vox-cdn.com/uploads/chorus_asset/file/25513843/2150185299.jpg?w=300&resize=300,300&ssl=1)














